
Publications
- English
- 日本語
Selected papers
-
Wayama, T.; Oguri, H. Michael-Addition-Triggered Release of Substituents from Tertiary Amines. Org. Lett. 2026, 28, 982–987. DOI: 10.1021/acs.orglett.5c04986.
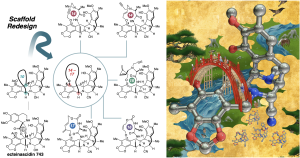
- Tanifuji, R.*; Hosono, E.; Kamakura, H.; Muramatsu, Y.; Yoshida, S.; Sato, S.; Ohashi, Y.; Dan, S.*; Seimiya, H.*; Oguri, H.* Strategic Scaffold Redesign of Ecteinascidins: An Approach for Generating Anticancer Macrocycles. Chem. 2025, 11, 102664. DOI: 10.1016/j.chempr.2025.102664.
-
Kiyoshi Shimohata, Mana Moriguchi, Masayuki Satake and Hiroki Oguri* “Programmable Divergence in Conia-ene and Hydroarylation Pathways for Collective Synthesis of Skeletally Diverse Alkaloid-like Scaffolds” ACS Catal. 2025, 15, 10991–11002.
https://doi.org/10.1021/acscatal.5c02364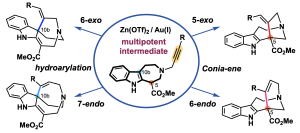
- Gavin Tay,‡ Soushi Nishimura‡ and Hiroki Oguri* Direct photochemical intramolecular [4 + 2] cycloadditions of dehydrosecodine-type substrates for the synthesis of the iboga-type scaffold and divergent [2 + 2] cycloadditions employing micro-flow system, Chem. Sci. 2024, 15, 15599–15609 (Edge Article).
https://pubs.rsc.org/en/content/articlelanding/2024/sc/d4sc02597k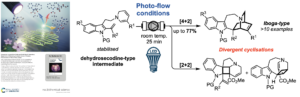
- Tasuku Honda, Daiji Ogata, Makoto Tsurui, Satoshi Yoshida, Sota Sato, Takahiro Muraoka,. Yuichi Kitagawa, Yasuchika Hasegawa,* Junpei Yuasa,* Hiroki Oguri* ”Rapid Synthesis of Chiral Figure-Eight Macrocycles Using a Preorganized Natural Product-Based Scaffold” Angew. Chem. Int. Ed. 2024, e202318548.
https://doi.org/10.1002/anie.202318548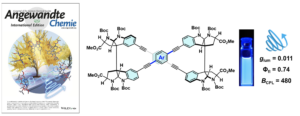
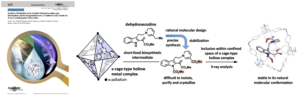
- Gavin Tay, Toshiaki Wayama, Hiroki Takezawa,* Satoshi Yoshida, Sota Sato,* Makoto Fujita,* Hiroki Oguri* ”Synthetic Modulation of an Unstable Dehydrosecodine-type Intermediate and Its Encapsulation into a Confined Cavity Enable Its X-ray Crystallographic Observation” Angew. Chem. Int. Ed. 2023, 62, e202305122 . https://doi.org/10.1002/ange.202305122
-
Toshiaki Wayama, Hiroki Oguri*
“Synthesis of a Halicyclamine-Type Macrocyclic Scaffold via Biomimetic Transannular Cyclization”
Org. Lett. 2023, 25, 3591–3595.
https://doi.org/10.1021/acs.joc.2c00212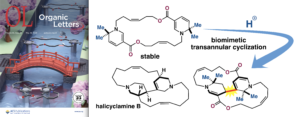
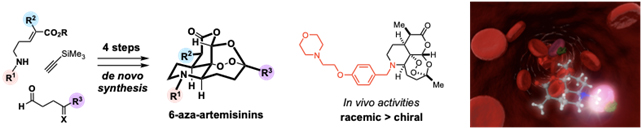
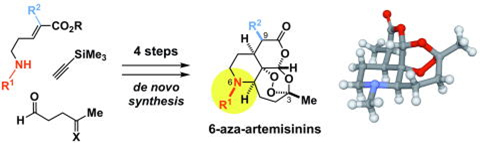
- Karunakar Reddy Bonepally, Norihito Takahashi, Naoya Matsuoka, Hikari Koi, Haruki Mizoguchi, Takahisa Hiruma, Kyohei Ochiai, Shun Suzuki, Yutaka Yamagishi, Hideaki Oikawa, Aki Ishiyama, Rei Hokari, Masato Iwatsuki,* Kazuhiko Otoguro, Satoshi Ōmura, Nobutaka Kato, Hiroki Oguri* “Rapid and Systematic Exploration of Chemical Space Relevant to Artemisinins: Anti-malarial Activities of Skeletally Diversified Tetracyclic Peroxides and 6-Aza-artemisinins” J. Org. Chem. 2020, 85, 9694-9712. https://doi.org/10.1021/acs.joc.0c01017
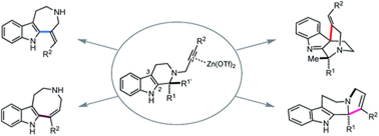
- Sadaiwa Yorimoto, Akira Tsubouchi, Haruki Mizoguchi, Hideaki Oikawa, Yoshiaki Tsunekawa, Tomoya Ichino, Satoshi Maeda,* Hiroki Oguri* “Zn(OTf)2-mediated annulations of N-propargylated tetrahydrocarbolines: divergent synthesis of four distinct alkaloidal scaffolds” Chemical Science 2019, 10, 5686-5698. https://doi.org/10.1039/C9SC01507H
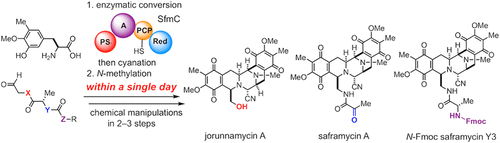
- Ryo Tanifuji, Kento Koketsu, Michiko Takakura, Ryutaro Asano, Atsushi Minami, Hideaki Oikawa*, Hiroki Oguri* “Chemo-enzymatic Total Syntheses of Jorunnamycin A, Saframycin A, and N-Fmoc Saframycin Y3″ J. Am. Chem. Soc. 2018, 140, 10705-10709. https://doi.org/10.1021/jacs.8b07161 Chem-station, spotlight research: https://www.chem-station.com/blog/2018/10/saframycin.html
- Karnakar Reddy Bonepally, Takahisa Hiruma, Haruki Mizoguchi, Kyohei Ochiai, Shun Suzuki, Hideaki Oikawa, Aki Ishiyama, Rei Hokari, Masato Iwatsuki, Kazuhiko Otoguro, Satoshi Ōmura, Hiroki Oguri* “Design and De Novo Synthesis of 6-Aza-Artemisinins” Org. Lett. 2018, 20, 4667-4671. https://doi.org/10.1021/acs.orglett.8b01987
- Haruki Mizoguchi, Hideaki Oikawa, Hiroki Oguri* “Biogenetically inspired synthesis and skeletal diversification of indole alkaloids” Nature Chemistry 2014, 6, 57-64. https://doi.org/10.1038/nchem.1798
- Hiroki Oguri,* Takahisa Hiruma, Yutaka Yamagishi, Hideaki Oikawa, Aki Ishiyama, Kazuhiko Otoguro, Haruki Yamada, Satoshi Ōmura “Generation of anti-trypanosomal agents through concise synthesis and structural diversification of sesquiterpene analogs” J. Am. Chem. Soc. 2011, 133, 7096-7105. Selected for the Front Cover https://doi.org/10.1021/ja200374q
- Kento Koketsu, Kenji Watanabe, Haruna Suda, Hiroki Oguri, Hideaki Oikawa* “Reconstruction of the saframycin core scaffold defines dual Pictet-Spengler mechanisms” Nature Chemical Biology 2010, 6, 408-410. https://doi.org/10.1038/nchembio.365
- Yoshihiro Shichijo, Akira Migita, Hiroki Oguri,* Mami Watanabe, Tetsuo Tokiwano, Kenji Watanabe, Hideaki Oikawa* “Epoxide Hydrolase Lsd19 for Polyether Formation in the Biosynthesis of Lasalocid A: Direct Experimental Evidence on Polyene-polyepoxide Hypothesis in Polyether Biosynthesis” J. Am. Chem. Soc. 2008, 130, 12230-12231. https://doi.org/10.1021/ja8040543
- Hiroki Oguri, Stuart L. Schreiber* “Skeletal Diversity via a Folding Pathway: Synthesis of Indole Alkaloid-Like Skeletons” Org. Lett. 2005, 7, 47-50. https://doi.org/10.1021/ol047945w
- Hiroki Oguri, Masahiro Hirama,* Takeshi Tsumuraya, Ikuo Fujii,* Megumi Maruyama, Hisatoshi Uehara, Yoko Nagumo “Synthesis-Based Approach toward Direct Sandwich Immunoassay for Ciguatoxin CTX3C” J. Am. Chem. Soc. 2003, 125, 7608-7612. https://doi.org/10.1021/ja034990a
- Masahiro Hirama,* Tohru Oishi, Hisatoshi Uehara, Masayuki Inoue, Megumi Maruyama, Hiroki Oguri, Masayuki Satake “Total Synthesis of Ciguatoxin CTX3C” Science 2001, 294, 1904-1907.https://doi.org/10.1126/science.1065757
- Masayuki Satake, Akio Morohashi, Hiroki Oguri, Tohru Oishi, Masahiro Hirama,* Nobuyuki Harada, Takeshi Yasumoto* “Absolute Configuration of Ciguatoxin” J. Am. Chem. Soc. 1997, 119, 11325-11326. https://doi.org/10.1021/ja972482t
Complete list
-
Murakami, S.; Honda, T.; Fushiki, T.; Yoshida, S.; Sato, S.; Toriumi, N.; Uchiyama, M.; Oguri, H. Dynamic Covalent Platform for Chiroptically Tunable Figure-Eight Macrocycles from Preorganized Alkaloidal Scaffolds. JACS Au, 2026, ASAP. DOI: 10.1021/jacsau.5c01493

-
Wayama, T.; Oguri, H. Michael-Addition-Triggered Release of Substituents from Tertiary Amines. Org. Lett. 2026, 28, 982–987. DOI: 10.1021/acs.orglett.5c04986.
- Kaneko, N.; Himeno, M.; Kobayashi, Y.; Tanifuji, R.; Kubota, H.; Mizoguchi, H.; Muroi, M.; Suzuki, T.; Sugiyama, M.; Dohmae, N.; Osada, H.; Kido, T.; Miyajima, A.; Oguri, H. RSC Chem. Biol. 2026, 7, 105–119. DOI: 10.1039/D5CB00268K.
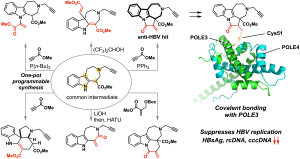
- Tanifuji, R.*; Hosono, E.; Kamakura, H.; Muramatsu, Y.; Yoshida, S.; Sato, S.; Ohashi, Y.; Dan, S.*; Seimiya, H.*; Oguri, H.* Strategic Scaffold Redesign of Ecteinascidins: An Approach for Generating Anticancer Macrocycles. Chem. 2025, 11, 102664. DOI: 10.1016/j.chempr.2025.102664.

-
Kiyoshi Shimohata, Mana Moriguchi, Masayuki Satake and Hiroki Oguri* “Programmable Divergence in Conia-ene and Hydroarylation Pathways for Collective Synthesis of Skeletally Diverse Alkaloid-like Scaffolds” ACS Catal. 2025, 15, 10991–11002.
https://doi.org/10.1021/acscatal.5c02364
- Hiroki Oguri* “Rapid and modular synthesis of skeletally diverse natural product analogs by expansion of biosynthetic processes” Bull. Chem. Soc. Jpn. 2025, 98, uoaf036.
https://academic.oup.com/bcsj/article/98/5/uoaf036/8114738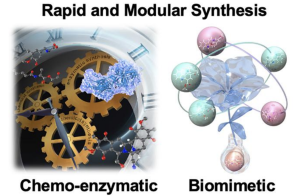
-
Asahi Kanno, Ryo Tanifuji,* Satoshi Yoshida, Sota Sato, Saori Maki-Yonekura, Kiyofumi Takaba, Jungmin Kang, Kensuke Tono, Koji Yonekura, and Hiroki Oguri* “Streamlined modular synthesis of saframycin substructure via copper-catalyzed three-component assembly and gold-promoted 6-endo cyclization” Beilstein J. Org. Chem. 2025, 21, 226–233.
https://www.beilstein-journals.org/bjoc/articles/21/14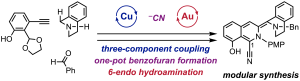
-
Gavin Tay,‡ Soushi Nishimura‡ and Hiroki Oguri* Direct photochemical intramolecular [4 + 2] cycloadditions of dehydrosecodine-type substrates for the synthesis of the iboga-type scaffold and divergent [2 + 2] cycloadditions employing micro-flow system, Chem. Sci. 2024, 15, 15599–15609 (Edge Article).
https://pubs.rsc.org/en/content/articlelanding/2024/sc/d4sc02597k
- Ryo Tanifuji,* Hiroki Oguri*, “Chemo-enzymatic total synthesis: current approaches toward the integration of chemical and enzymatic transformations” Beilstein J. Org. Chem. 2024, 20, 1693–1712.
https://www.beilstein-journals.org/bjoc/articles/20/151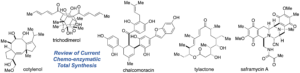
- Tasuku Honda, Daiji Ogata, Makoto Tsurui, Satoshi Yoshida, Sota Sato, Takahiro Muraoka,. Yuichi Kitagawa, Yasuchika Hasegawa,* Junpei Yuasa,* Hiroki Oguri* ”Rapid Synthesis of Chiral Figure-Eight Macrocycles Using a Preorganized Natural Product-Based Scaffold” Angew. Chem. Int. Ed. 2024, e202318548.
https://doi.org/10.1002/anie.202318548
- Gavin Tay, Toshiaki Wayama, Hiroki Takezawa,* Satoshi Yoshida, Sota Sato,* Makoto Fujita,* Hiroki Oguri* ”Synthetic Modulation of an Unstable Dehydrosecodine-type Intermediate and Its Encapsulation into a Confined Cavity Enable Its X-ray Crystallographic Observation” Angew. Chem. Int. Ed. 2023, 62, e202305122.
https://doi.org/10.1002/ange.202305122
- Toshiaki Wayama, Hiroki Oguri*
“Synthesis of a Halicyclamine-Type Macrocyclic Scaffold via Biomimetic Transannular Cyclization”
Org. Lett. 2023, 25, 3591–3595.
https://doi.org/10.1021/acs.joc.2c00212
- Toshiaki Wayama, Yuta Arai, Hiroki Oguri*
“Regiocontrolled Dimerization of Densely Functionalized 1,6-Dihydropyridines for the Biomimetic Synthesis of a Halicyclamine-type Scaffold by Preventing Disproportionation”
J. Org. Chem. 2022, 87, 5938–5951.
https://doi.org/10.1021/acs.joc.2c00212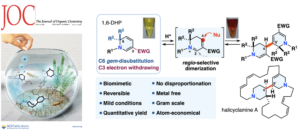
- Ryo Tanifuji*, Naoto Haraguchi, Hiroki Oguri*
“Chemo-enzymatic total syntheses of bis-tetrahydroisoquinoline alkaloids and systematic exploration of the substrate scope of SfmC”
Tetrahedron Chem. 2022, 1, 100010.
https://doi.org/10.1016/j.tchem.2022.100010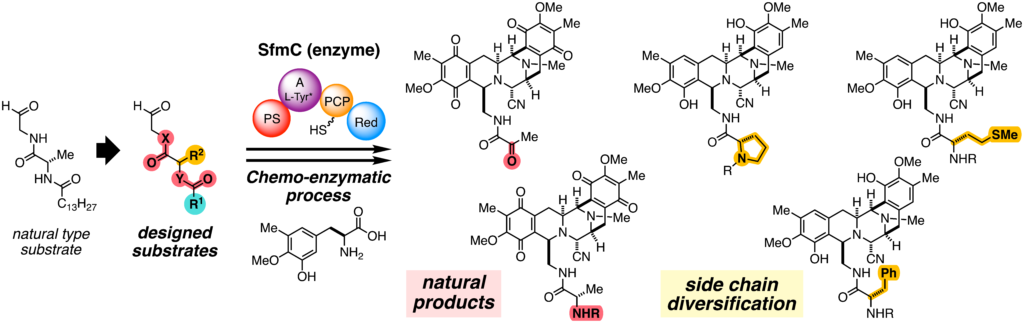
- Yuta Arai, Hiroki Oguri*
“Divergent synthesis of functionalized dihydropyridines and pyrroles via metal-free one-pot domino reactions of a gem-disubstituted propargyl amine and an alkynyl sulfone”
Tetrahedron Lett. 2021, 78, 153283.
https://doi.org/10.1016/j.tetlet.2021.153283 - Hiroki Oguri*
“Synthesis and Structural Diversification of Artemisinins Towards the Generation
of Potent Anti-malarial Agents”
Chem. Lett. 2021, Invited contribution for Vol. 50 commemorative highlight review, in press
https://doi.org/10.1246/cl.200920 - Takahiro Shimizu, Norihito Takahashi, Vincent J. Huber, Yasunobu Asawa, Hiroki Ueda, Atsushi Yoshimori, Yukiko Muramatsu, Hiroyuki Seimiya, Hiroyuki Kouji, Hiroyuki Nakamura, Hiroki Oguri*
“Design and synthesis of 14 and 15-membered macrocyclic scaffolds exhibiting inhibitory activities of hypoxia-inducible factor 1”
Bioorg. Med. Chem. 2021, 30, 115949.
https://doi.org/10.1016/j.bmc.2020.115949 - Karunakar Reddy Bonepally, Norihito Takahashi, Naoya Matsuoka, Hikari Koi, Haruki Mizoguchi, Takahisa Hiruma, Kyohei Ochiai, Shun Suzuki, Yutaka Yamagishi, Hideaki Oikawa, Aki Ishiyama, Rei Hokari, Masato Iwatsuki, Kazuhiko Otoguro, Satoshi Ōmura, Nobutaka Kato, Hiroki Oguri*
“Rapid and Systematic Exploration of Chemical Space Relevant to Artemisinins: Anti-malarial Activities of Skeletally Diversified Tetracyclic Peroxides and 6-Aza-artemisinins”
J. Org. Chem. 2020, 85, 9694-9712.
https://doi.org/10.1021/acs.joc.0c01017 - Hikari Koi, Norihito Takahashi, Yasufumi Fuchi, Tomohiro Umeno, Yukiko Muramatsu, Hiroyuki Seimiya, Satoru Karasawa, and Hiroki Oguri*
“A fully synthetic 6-aza-artemisinin bearing an amphiphilic chain generates aggregates and exhibits anti-cancer activities”
Org. Biomol. Chem. 2020, 18, 5339?5343.
https://doi.org/10.1039/D0OB00919A - Ryo Tanifuji, Atsushi Minami, Hiroki Oguri * and Hideaki Oikawa*
“Total synthesis of alkaloids using both chemical and biochemical methods”
Nat. Prod. Rep. 2020, 37, 1098-1121.
https://doi.org/10.1039/C9NP00073A - Sadaiwa Yorimoto, Akira Tsubouchi, Haruki Mizoguchi, Hideaki Oikawa, Yoshiaki Tsunekawa, Tomoya Ichino, Satoshi Maeda and Hiroki Oguri
“Zn(OTf)2-mediated annulations of N-propargylated tetrahydrocarbolines: divergent synthesis of four distinct alkaloidal scaffolds”
Chemical Science 2019, 10, 5686-5698.
https://doi.org/10.1039/C9SC01507H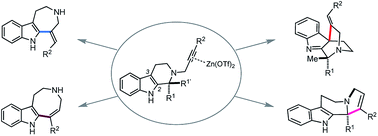
- Ryo Tanifuji, Kaori Tsukakoshi, Kazunori Ikebukuro, Hideaki Oikawa, Hiroki Oguri
“Generation of C5-desoxy analogs of tetrahydroisoquinoline alkaloids exhibiting potent DNA alkylating ability”
Bioorg. Med. Chem. Lett. 2019, 29, 1807-1811.
https://doi.org/10.1016/j.bmcl.2019.05.009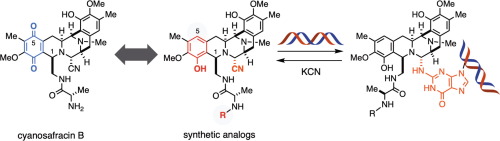
- Mitsuhiro Wada, Hiroyuki Suzuki, Mitsuyasu Kato, Hideaki Oikawa, Akira Tsubouchi, and Hiroki Oguri
“Stereo-Divergent Synthesis of Bispyrrolidinoindoline Alkaloidal Scaffolds and Generation of a Lead Candidate with Stereospecific Anti-proliferative Activity”
ChemBioChem. 2019, 20, 1273-1281. in press As an invited contribution to the special issue “ChembioTalents”, Selected as the back cover article.
https://doi.org/10.1002/cbic.201800815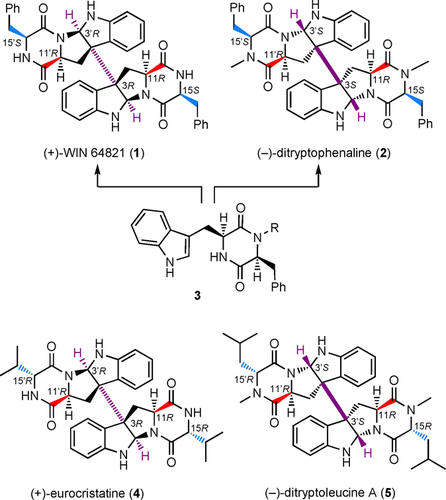
- Tomoaki Taniguchi, Akira Tsubouchi, Yuki Imai, Junpei Yuasa, Hiroki Oguri
“Chiroptical Inversion of Europium(III) Complexes by Changing a Remote Stereogenic Center of a C2-symmetric Bispyrrolidinoindoline Manifold”
J. Org .Chem. 2018, 83, 24, 15284?15296.
https://doi.org/10.1021/acs.joc.8b02550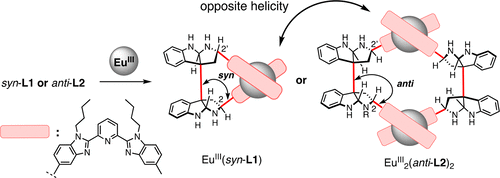
- Nana Tsuchiya, Yunosuke Ryu, Takahiro Muraoka, Hiroki Oguri
“Design of C2-symmetric alkaloidal chiral amphiphiles and configurational effects on self-assembly”
Org. Biomol. Chem. 2018, 16, 9305-9313. Selected as the front cover article.
https://doi.org/10.1039/C8OB02287A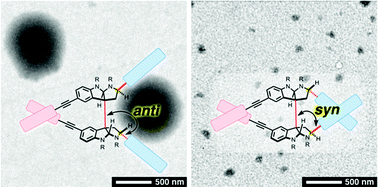
- Ryo Tanifuji, Kento Koketsu, Michiko Takakura, Ryutaro Asano, Atsushi Minami, Hideaki Oikawa*, and Hiroki Oguri*
“Chemo-enzymatic Total Syntheses of Jorunnamycin A, Saframycin A, and N-Fmoc Saframycin Y3“
J. Am. Chem. Soc. 2018, 140, 10705-10709.
https://doi.org/10.1021/jacs.8b07161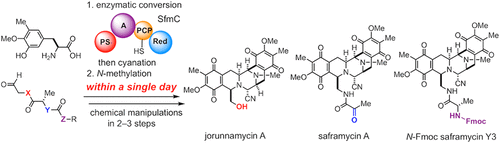
- Karnakar Reddy Bonepally, Takahisa Hiruma, Haruki Mizoguchi, Kyohei Ochiai, Shun Suzuki, Hideaki Oikawa, Aki Ishiyama, Rei Hokari, Masato Iwatsuki, Kazuhiko Otoguro, Satoshi Omura, and Hiroki Oguri*
“Design and De Novo Synthesis of 6-Aza-Artemisinins”
Org. Lett. 2018, 20, 4667?4671.
https://doi.org/10.1021/acs.orglett.8b01987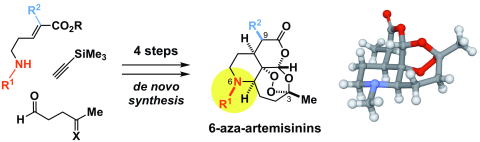
- Ryo Watanabe, Haruki Mizoguchi, Hideaki Oikawa, Hirofumi Ohashi, Koichi Watashi, and Hiroki Oguri*
“Stereo-controlled synthesis of functionalized tetrahydropyridines based on the cyanomethylation of 1,6-dihydropyridines and generation of anti-hepatitis C virus agents”
Bioorg. Med. Chem. 2017, 25, 2851-2855.
https://doi.org/10.1016/j.bmc.2017.03.011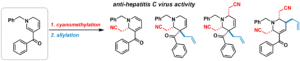
- Ryo Tanifuji, Hiroki Oguri,* Kento Koketsu, Yuki Yoshinaga, Atsushi Minami, Hideaki Oikawa*
“Catalytic asymmetric synthesis of the common amino acid component in the biosynthesis of tetrahydroisoquinoline alkaloids”
Tetrahedron Lett. 2016, 57, 623-626.
https://doi.org/10.1016/j.tetlet.2015.12.110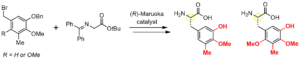
- Hiroki Oguri*
“Biomimetic assembly lines producing natural product analogs: Strategies from a versatile manifold to skeletally diverse scaffolds”
Chemical Record 2016, 16, 652-666.
https://doi.org/10.1002/tcr.201500213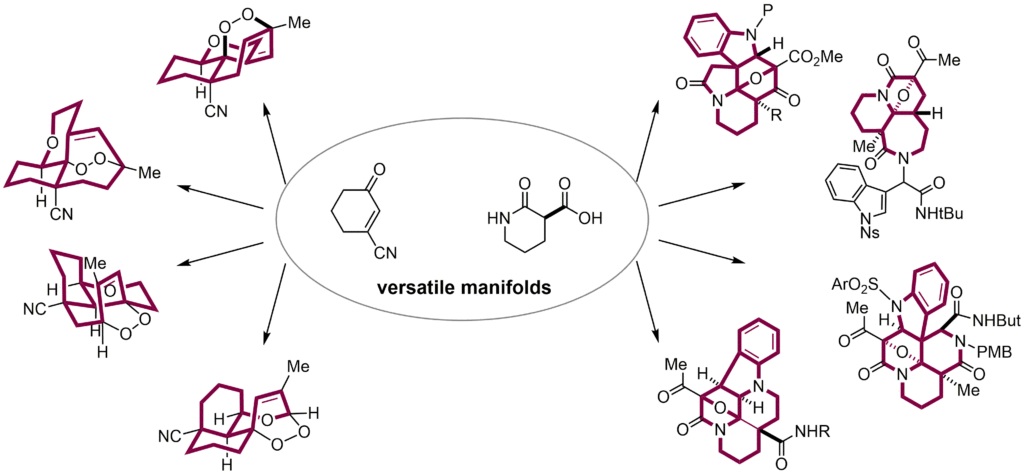
- Haruki Mizoguchi, Ryo Watanabe, Shintaro Minami, Hideaki Oikawa, Hiroki Oguri*
“Synthesis of multiply substituted 1,6-dihydropyridines through Cu(I)-catalyzed 6-endo cyclization”
Org. Biomol. Chem. 2015,13, 5955?5963.
https://doi.org/10.1039/C5OB00356C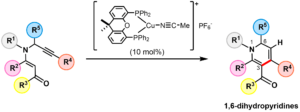
- Takahiro Ugai, Atsushi Minami, Ryuya Fujii, Mizuki Tanaka, Hiroki Oguri, Katsuya Gomi, Hideaki Oikawa*
“Heterologous expression of highly reducing polyketide synthase involved in betaenone biosynthesis”
Chem. Commun. 2015, 51, 1878-1881.
https://doi.org/10.1039/C4CC09512J - Gaku Suzuki, Atsushi Minami, Mayu Shimaya, Takeshi Kodama, Yoshiki Morimoto, Hiroki Oguri, Hideaki Oikawa*
“Analysis of enantiofacial selective epoxidation catalyzed by flavin-containing monooxygenase Lsd18 involved in ionophore polyether lasalocid biosynthesis”
Chem. Lett. 2014, 43, 1779-1781.
https://doi.org/10.1246/cl.140721 - Haruki Mizoguchi, Hideaki Oikawa, Hiroki Oguri*
“Biogenetically inspired synthesis and skeletal diversification of indole alkaloids”
Nature Chemistry 2014, 6, 57-64.
https://doi.org/10.1038/nchem.1798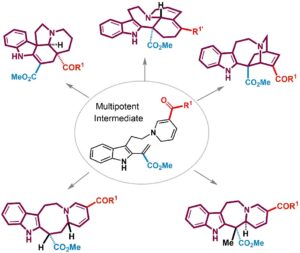
- Atsushi Minami, Toyoyuki Ose, Kyohei Sato, Azusa Oikawa, Kimiko Kuroki, Katsumi Maenaka, Hiroki Oguri, Hideaki Oikawa*
“Allosteric regulation of epoxide opening cascades by a pair of epoxide hydrolases in monensin biosynthesis”
ACS Chem. Biol. 2014, 9, 562?569.
https://doi.org/10.1021/cb4006485 - Gaku Suzuki, Atsushi Minami, Mayu Shimaya, Takeshi Kodama, Yoshiki Morimoto, Hiroki Oguri, and Hideaki Oikawa*
“Analysis of Enantiofacial Selective Epoxidation Catalyzed by Flavin-containing Monooxygenase Lsd18 Involved in Ionophore Polyether Lasalocid Biosynthesis”
Chem. Lett. 2014, 43, 1779-1781.
https://doi.org/10.1246/cl.140721 - Mitsuhiro Wada, Takahisa Murata, Hideaki Oikawa, Hiroki Oguri*
“A nickel-catalyzed dimerization of pyrrolidinoindoline scaffold: a systematic access to chimonanthines, folicanthines and (+)-WIN 64821”
Org. Biomol. Chem. 2014, 12, 298-306.
https://doi.org/10.1039/C3OB41918E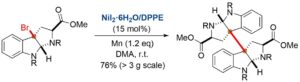
- Tomoshige Hiratsuka, Kento Koketsu, Atsushi Minami, Shunsuke Kaneko, Chiaki Yamazaki, Kenji Watanabe, Hiroki Oguri, Hideaki Oikawa*
“Core Assembly Mechanism of Quinocarcin/SF-1739: Bimodular Complex Nonribosomal Peptide Synthetases for Sequential Mannich-type Reactions”
Chem. Biol. 2013, 20, 1523-1535.
https://doi.org/10.1016/j.chembiol.2013.10.011 - Atsushi Minami, Hiroki Oguri, Kenji Watanabe, Hideaki Oikawa*
“Biosynthetic machinery of ionophore polyether lasalocid: enzymatic construction of polyether skeleton”
Curr. Opin. Chem. Biol. 2013, 17, 555-561.
https://doi.org/10.1016/j.cbpa.2013.06.004 - Velisoju Mahendar, Hideaki Oikawa, Hiroki Oguri*
“Sequential [6+2], [2+2], [3+2] Annulations for Rapid Assembly of Multiple Fragments”
Chem. Commun. 2013, 49, 2299-2301.
https://doi.org/10.1039/C2CC38854E - Kento Koketsu, Atsushi Minami, Kenji Watanabe, Hiroki Oguri, Hideaki Oikawa*
“The Pictet-Spengler Mechanism Involved in the Biosynthesis of Tetrahydroisoquinoline Antitumor Antibiotics: A Novel Function for a Nonribosomal Peptide Synthetase”
Methods Enzymol. 2012, 516, 79-98.
https://doi.org/10.1016/B978-0-12-394291-3.00026-5 - Hiroki Oguri,* Haruki Mizoguchi, Hideaki Oikawa, Aki Ishiyama, Masato Iwatsuki, Kazuhiko Otoguro, Satoshi Ōmura
“Parallel and four-step synthesis of natural product-inspired scaffolds through modular assembly and divergent cyclization”
Beilstein J. Org. Chem. 2012, 8, 930-940. (Thematic Issue: Recent Developments in Chemical Diversity)
https://doi.org/10.3762/bjoc.8.105 - Atsushi Minami, Mayu Shimaya, Gaku Suzuki, Akira Migita, Sandip S. Shinde, Kyohei Sato, Kenji Watanabe, Tomohiro Tamura, Hiroki Oguri, Hideaki Oikawa*
“Sequential enzymatic epoxidation involved in polyether lasalocid biosynthesis”
J. Am. Chem. Soc. 2012, 134, 7246-7249.
https://doi.org/10.3762/bjoc.8.105 - Haruki Mizoguchi, Hideaki Oikawa, Hiroki Oguri*
“Hg(OTf)2-Catalyzed Direct Vinylation of Tryptamines and Versatile Applications for Tandem Reactions”
Org. Biomol. Chem. 2012, 10, 4236-4242.
https://doi.org/10.1039/C2OB25236H - Kento Koketsu, Atsushi Minami, Kenji Watanabe, Hiroki Oguri, Hideaki Oikawa*
“Pictet-Spenglerase involved in tetrahydroisoquinoline antibiotic biosynthesis”
Curr. Opin. Chem. Biol. 2012, 16, 142-149.
https://doi.org/10.1016/j.cbpa.2012.02.021 - Kyohei Sato, Atsushi Minami, Toyoyuki Ose, Hiroki Oguri, Hideaki Oikawa*
“Remarkable synergistic effect between MonBI and MonBII on epoxide openings reaction in ionophore polyether monensin biosynthesis”
Tetrahedron Lett. 2011, 52, 5277-5280.
https://doi.org/10.1016/j.tetlet.2011.07.145 - Hyun Kim, Chie Nakajima, Kazumasa Yokoyama, Zeaur Rahim, Youn Uck Kim, Hiroki Oguri, Yasuhiko Suzuki*
“Impact of the E540V amino acid substitution in GyrB of Mycobacterium tuberculosis Quinolone Resistance”
Antimicrobial Agents and Chemotherapy 2011, 55, 3661-3667.
https://doi.org/10.1128/AAC.00042-11 - Hiroki Oguri,* Takahisa Hiruma, Yutaka Yamagishi, Hideaki Oikawa, Aki Ishiyama, Kazuhiko Otoguro, Haruki Yamada, Satoshi Ōmura
“Generation of anti-trypanosomal agents through concise synthesis and structural diversification of sesquiterpene analogs”
J. Am. Chem. Soc. 2011, 133, 7096-7105. Selected for the Front Cover
https://doi.org/10.1021/ja200374q - Atsushi Minami, Akira Migita, Daiki Inada, Kinya Hotta, Kenji Watanabe, Hiroki Oguri, Hideaki Oikawa*
“Enzymatic epoxide-opening cascades catalyzed by a pair of epoxide hydrolases in the ionophore polyether biosynthesis”
Org. Lett. 2011, 13, 1638-1641.
https://doi.org/10.1021/ol200100e - Yuki Hirose, Kenji Watanabe, Atsushi Minami, Takemichi Nakamura, Hiroki Oguri, Hideaki Oikawa*
“Involvement of Common Intermediate 3-Hydroxy-L-kyurenine in Chromophore Biosynthesis of Quinomycin Family Antibiotics”
J. Antibiot. 2011, 64, 117-122.
https://doi.org/10.1038/ja.2010.142 - Yusuke Matsuura, Yoshihiro Shichijo, Atsushi Minami, Akira Migita, Hiroki Oguri, Mami Watanabe, Tetsuo Tokiwano, Kenji Watanabe, Hideaki Oikawa*
“Intriguing Substrate Specificity of Epoxide Hydrolase Lad19 Involved in Biosynthesis of Ionophore Antibiotic Lasalocid A”
Org. Lett. 2010, 12, 2226-2229.
https://doi.org/10.1021/ol100541e - Ken Kasahara, Takanori Miyamato, Takashi Fujimoto, Hiroki Oguri, Tetsuo Tokiwano, Hideaki Oikawa, Yutaka Ebizuka, Isao Fujii*
“Solanapyrone synthase, a possible Diels-Alderase, and iterative type I polyketide synthase encoded in a biosynthetic gene cluster from Alternaria solani”
ChemBioChem 2010, 11, 1245-1252.
https://doi.org/10.1002/cbic.201000173 - Yuhei Ishigaki, Velisoju Mahendar, Hiroki Oguri,* Hideaki Oikawa
“An anti-tetraamination of a 1,3-diene unit via cascade annulations of the azulenone scaffold with dicarbonyl azo-compounds”
Chem. Commun. 2010, 46, 3304-3305.
https://doi.org/10.1039/B926676C - Kento Koketsu, Kenji Watanabe, Haruna Suda, Hiroki Oguri, Hideaki Oikawa*
“Reconstruction of the saframycin core scaffold defines dual Pictet-Spengler mechanisms”
Nature Chemical Biology 2010, 6, 408-410.
https://doi.org/10.1038/nchembio.365 - Kenji Watanabe, Hiroki Oguri, Hideaki Oikawa*
“Enzymatic Synthesis of Molecular Skeletons of Complex Antitumor Antibiotics with Non-ribosomal Peptide Synthetases”
J. Synth. Org. Chem., Jpn. 2009, 67(11), 1152-1160.
https://doi.org/10.5059/yukigoseikyokaishi.67.1152 - Haruki Mizoguchi, Hiroki Oguri,* Kiyoshi Tsuge, Hideaki Oikawa
“Divergent and Expeditious Access to Fused Skeletons Inspired by Indole Alkaloids and Transtaganolides”
Org. Lett. 2009, 11, 3016-3019.
https://doi.org/10.1021/ol901020a - Kenji Watanabe,* Kinya Hotta, Mino Nakaya, Alex P. Praseuth, Clay C. C. Wang, Daiki Inada, Kosaku Takahashi, Eri Fukushi, Hiroki Oguri, Hideaki Oikawa*
“Escherichia coli allows efficient modular incorporation of newly isolated quinomycin biosynthetic enzyme into echinomycin biosynthetic pathway for rational design and synthesis of potent antibiotic unnatural natural product”
J. Am. Chem. Soc. 2009, 131, 9347-9353.
https://doi.org/10.1021/ja902261a - Kenji Watanabe,* Kinya Hotta, Alex P. Praseuth, Mark Searcey, M, Clay C. C. Wang, Hiroki Oguri, Hideaki Oikawa
“Rationally Engineered Total Biosynthesis of a Synthetic Analog of a Natural Quinomycin Depsipeptide in Escherichia coli”
ChemBioChem 2009, 12, 1965-1968.
https://doi.org/10.1002/cbic.200900260 - Kenji Watanabe,* Hiroki Oguri, Hideaki Oikawa*
“Diversification of echinomycin molecular structure by way of chemoenzymatic synthesis and heterologous expression of the engineered echinomycin biosynthetic pathway”
Curr. Opin. Chem. Biol. 2009, 13, 189-196.
https://doi.org/10.1016/j.cbpa.2009.02.036 - Hiroki Oguri,* Yutaka Yamagishi, Takahisa Hiruma, Hideaki Oikawa
“Skeletal and Stereochemical Diversification of Tricyclic Frameworks Inspired by Ca2+-ATPase Inhibitors, Artemisinin and Transtaganolide D”
Org. Lett. 2009, 11, 601-604.
https://doi.org/10.1021/ol802621u - Alex P. Praseuth, Clay C. C. Wang,* Kenji Watanabe,* Kinya Hotta, Hiroki Oguri, Hideaki Oikawa
“Complete Sequence of Biosynthetic Gene Cluster Responsible for Producing Triostin A and Evaluation of Quinomycin-Type Antibiotics from Streptomyces triostinicus”
Biotechnol. Prog. 2008, 24, 1226-1231.
https://doi.org/10.1002/btpr.34 - Naoki Sugano, Yuuki Koizumi, Go Hirai, Hiroki Oguri, Shoji Kobayashi, Shuji Yamashita, Masahiro Hirama*
“Enantioselective Synthesis of the Fully Functionalized ABC Ring of Zoanthenol”
Chem. Asian J. 2008, 3, 1549-1557.
https://doi.org/10.1002/asia.200800079 - Yoshihiro Shichijo, Akira Migita, Hiroki Oguri,* Mami Watanabe, Tetsuo Tokiwano, Kenji Watanabe, Hideaki Oikawa*
“Epoxide Hydrolase Lsd19 for Polyether Formation in the Biosynthesis of Lasalocid A: Direct Experimental Evidence on Polyene-polyepoxide Hypothesis in Polyether Biosynthesis”
J. Am. Chem. Soc. 2008, 130, 12230-12231.
https://doi.org/10.1021/ja8040543 - Kento Koketsu, Hiroki Oguri,* Kenji Watanabe, Hideaki Oikawa*
“Enzymatic macrolactonization in the presence of DNA leading to triostin A analogs”
Chemistry & Biology 2008, 15, 818-828.
https://doi.org/10.1016/j.chembiol.2008.05.022 - Kouhei Tsumoto,* Akiko Yokota, Yoshikazu Tanaka, Mihoko Ui, Takeshi Tsumuraya, Ikuo Fujii, Izumi Kumagai, Yoko Nagumo, Hiroki Oguri, Masayuki Inoue, Masahiro Hirama
“Critical contribution of aromatic rings to specific recognition of polyether rings: The case of ciguatoxin CTX3C-ABC and its specific antibody 1C49”
J. Biol. Chem. 2008, 283, 12259-12266.
https://doi.org/10.1074/jbc.m710553200 - Akira Migita, Yoshihiro Shichijo, Hiroki Oguri,* Mami Watanabe, Tetsuo Tokiwano, Hideaki Oikawa*
“Stereo-controlled synthesis of prelasalocid, a key precursor proposed in the biosynthesis of polyether antibiotic lasalocid A”
Tetrahedron Lett. 2008, 49, 1021-1025.
https://doi.org/10.1016/j.tetlet.2007.12.003 - Alex P. Praseuth, Mike B. Praseuth, Hiroki Oguri, Hideaki Oikawa, Kenji Watanabe,* Clay C. C. Wang*
“Improved Production of Triostin A in Engineered Escherichia coli with Furnished Quinoxaline Chromophore by Design of Experiments in Small-Scale Culture”
Biotechnol. Prog. 2008, 24, 134-139.
https://doi.org/10.1021/bp070298y - Mino Nakaya, Hiroki Oguri, Kosaku Takahashi, Eri Fukushi, Kenji Watanabe, Hideaki Oikawa*
“Relative and absolute configuration of antitumor agent SW-163D”
Biosci. Biotechnol. Biochem. 2007, 71, 2969-2976.
https://doi.org/10.1271/bbb.70371 - Hiroki Oguri*
“Bioorganic Studies Utilizing Rationally Designed Synthetic Molecules: Absolute Configuration of Ciguatoxin and Development of Immunoassay Systems”
Bull. Chem. Soc. Jpn. 2007, 80, 1870-1883.
https://doi.org/10.1246/bcsj.80.1870 - Kento Koketsu, Hiroki Oguri,* Kenji Watanabe, Hideaki Oikawa*
“Identification and Stereochemical Assignment of the β-Hydroxytryptophan Intermediate in the Echinomycin Biosynthetic Pathway”
Org. Lett. 2006, 8, 4719-4722.
https://doi.org/10.1021/ol061738+ - Kenji Watanabe,* Kinya Hotta, Alex P. Praseuth, Kento Koketsu, Akira Migita, Christopher N. Boddy, Clay C. C. Wang, Hiroki Oguri, Hideaki Oikawa*
“Total Biosynthesis of Antitumor Nonribosomal Peptide in Escherichia coli”
Nature Chemical Biology 2006, 2, 423-428.
https://doi.org/10.1038/nchembio803 - Hiroki Oguri,* Shintaro Tanabe, Akifumi Oomura, Mitsuo Umetsu, Masahiro Hirama*
“Synthesis and evaluation of alpha-helix mimetics based on a trans-fused polycyclic ether: sequence-selective binding to aspartate pairs in alpha-helical peptides”
Tetrahedron Lett. 2006, 47, 5801-5805.
https://doi.org/10.1016/j.tetlet.2006.05.170 - Hiroki Oguri, Stuart L. Schreiber*
“Skeletal Diversity via a Folding Pathway: Synthesis of Indole Alkaloid-Like Skeletons”
Org. Lett. 2005, 7, 47-50.
https://doi.org/10.1021/ol047945w - Yuta Fujita, Hiroki Oguri, Hideaki Oikawa*
“Biosynthetic Studies on the antibiotics PF1140: a novel pathway for a 2-pyridone framework”
Tetrahedron Lett. 2005, 46, 5885-5888.
https://doi.org/10.1016/j.tetlet.2005.06.115 - Yuta Fujita, Hiroki Oguri, Hideaki Oikawa*
“The Relative and Absolute Configuration of PF1140”
J. Antibiot. 2005, 58, 425-427.
https://doi.org/10.1038/ja.2005.56 - Hiroki Oguri,* Akifumi Oomura, Shintaro Tanabe, Masahiro Hirama*
“Design and synthesis of a trans-fused polycyclic ether skeleton as an alpha-helix mimetic scaffold”
Tetrahedron Lett. 2005, 46, 2179-2183.
https://doi.org/10.1016/j.tetlet.2005.02.039 - Yoko Nagumo, Hiroki Oguri,* Kouhei Tsumoto,* Yumi Shindo, Masahiro Hirama,* Takeshi Tsumuraya, Ikuo Fujii,* Yoshihisa Tomoika, Michinao Mizugaki, Izumi Kumagai
“Phage-Display Selection of Antibodies to Left End of CTX3C Using Synthetic Fragments”
J. Immunol. Methods 2004, 289, 137-146.
https://doi.org/10.1016/j.jim.2004.04.003 - Go Hirai, Hiroki Oguri,* Masahiko Hayashi, Koji Koyama, Yuuki Koizumi, Sameh M. Moharram, Masahiro Hirama*
“Synthesis and preliminary biological evaluation of truncated zoanthenol analogues”
Bioorg. Med. Chem. Lett. 2004, 14, 2647-2651.
https://doi.org/10.1016/j.bmcl.2004.02.064 - Shoji Kobayashi, Babak, H. Alizadeh, Shin-ya Sasaki, Hiroki Oguri, Masahiro Hirama*
“Synthesis of the Fully Functionalized ABCDE Ring Moiety of Ciguatoxin”
Org. Lett. 2004, 5, 751-754.
https://doi.org/10.1021/ol0364475 - Hiroki Oguri, Masahiro Hirama,* Takeshi Tsumuraya, Ikuo Fujii,* Megumi Maruyama, Hisatoshi Uehara, Yoko Nagumo
“Synthesis-Based Approach toward Direct Sandwich Immunoassay for Ciguatoxin CTX3C”
J. Am. Chem. Soc. 2003, 125, 7608-7612.
https://doi.org/10.1021/ja034990a - Jin Wang, Satoshi Sakamoto, Kei Kamada, Aiko Nitta, Takeshi Noda, Hiroki Oguri, Masahiro Hirama*
“Construction of the AG-ring unit of pinnatoxin A via intramolecular alkylation and aza-wittig reaction”
Synlett 2003, 891-893.
https://doi.org/10.1055/s-2003-38752 - Lilian Rumi Tsuruta, Yoshihisa Tomioka, Takanori Hishinuma, Yoshinori Kato, Kunihiko Itoh, Toshio Suzuki, Hiroki Oguri, Masahiro Hirama, Junichi Goto,* Michinao Mizugaki
“Characterization of 11-dehydro-thromboxane B2 recombinant antibody obtained by phage display technology”
Prostaglandins, Leukotrienes and Essential Fatty Acids 2003, 68, 273-284.
https://doi.org/10.1016/S0952-3278(03)00006-1 - Sameh M. Moharram, Hiroki Oguri, Masahiro Hirama*
“Coupling of A-C ring moieties of zoanthenol alkaloids based on Suzuki-Miyaura coupling reaction”
Egypt. J. Pham. Sci. 2003, 44, 177-193. - Go Hirai, Yuuki Koizumi , Sameh M. Moharram, Hiroki Oguri, Masahiro Hirama*
“Construction of the Benzylic Quaternary Carbon Center of Zoanthenol by Intramolecular Mizoroki-Heck Reaction of Enone”
Org. Lett. 2002, 4, 1627-1630.
https://doi.org/10.1021/ol025852d - Megumi Maruyama, Masayuki Inoue, Tohru Oishi, Hiroki Oguri, Yoshihiro Ogasawara, Yumi Shindo, Masahiro Hirama*
“Convergent synthesis of the ABCDE Ring Fragment of Ciguatoxin CTX3C”
Tetrahedron 2002, 58, 1835-1851.
https://doi.org/10.1016/S0040-4020(02)00041-8 - Masahiro Hirama,* Tohru Oishi, Hisatoshi Uehara, Masayuki Inoue, Megumi Maruyama, Hiroki Oguri, Masayuki Satake
“Total Synthesis of Ciguatoxin CTX3C”
Science 2001, 294, 1904-1907.
https://doi.org/10.1126/science.1065757 - Yoko Nagumo, Hiroki Oguri, Yumi Shindo, Shin-ya Sasaki, Tohru Oishi, Masahiro Hirama,* Yoshihisa Tomioka, Michinao Mizugaki, Takeshi Tsumuraya
“Concise synthesis of ciguatoxin ABC-ring fragments and surface plasmon resonance study of the interaction of their BSA conjugates with monoclonal antibodies”
Bioorg. Med. Chem. Lett. 2001,15, 2037-2040.
https://doi.org/10.1016/S0960-894X(01)00358-4 - Go Hirai, Hiroki Oguri, Sameh M. Moharram, Koji Koyama, Masahiro Hirama*
“Convergent synthesis of the ABC-ring moiety of zoanthenol: intramolecular Mizoroki-Heck reaction”
Tetrahedron Lett. 2001, 42, 5783-5787.
https://doi.org/10.1016/S0040-4039(01)01110-8 - Hiroto Imai, Hisatoshi Uehara, Masayuki Inoue, Hiroki Oguri, Tohru Oishi, Masahiro Hirama*
“Convergent synthesis of the EFGH ring fragment of ciguatoxin CTX3C”
Tetrahedron Lett. 2001, 42, 6219-6222.
https://doi.org/10.1016/S0040-4039(01)01219-9 - Megumi Maruyama, Kenji Maeda, Tohru Oishi, Hiroki Oguri, Masahiro Hirama*
“Convergent Strategy for Synthesizing Polycyclic Ether Marine Toxins: Synthesis of the ABCDE Ring Fragment of Ciguatoxin 3C”
Heterocycles 2001, 54, 93-99.
https://doi.org/10.3987/COM-00-S(I)61 - Tohru Oishi, Shin-ichiro Tanaka, Yoshihiro Ogasawara, Kenji Maeda, Hiroki Oguri, Masahiro Hirama*
“Highly Stereocontrolled Synthesis of the ABCD Ring Fragment of Ciguatoxin CTX3C”
Synlett 2001, 952-954.
https://doi.org/10.1055/s-2001-14631 - Hiroki Oguri, Shin-ichiro Tanaka, Tohru Oishi, Masahiro Hirama*
“A Very Short Route to the Functionalized A-ring Moiety of Ciguatoxin”
Tetrahedron Lett. 2000, 41, 975-978.
https://doi.org/10.1016/S0040-4039(99)02185-1 - Sameh M. Moharram, Go Hirai, Koji Koyama, Hiroki Oguri, Masahiro Hirama*
“Enantio-Face Control by Molecular Sieves in the Asymmetric Diels-Alder Reaction”
Tetrahedron Lett. 2000, 41, 6669-6673.
https://doi.org/10.1016/S0040-4039(00)01116-3 - Hiroki Oguri, Shin-ya Sasaki, Tohru Oishi, Masahiro Hirama*
“Expeditious Tandem-Metathesis Route to the AB-ring Moiety of Ciguatoxin”
Tetrahedron Lett. 1999, 40, 5405-5408.
https://doi.org/10.1016/S0040-4039(99)01017-5 - Kenji Maeda, Tohru Oishi, Hiroki Oguri, Masahiro Hirama*
“Convergent Synthesis of the ABCDE-ring Framework of Ciguatoxin”
Chem. Commun. 1999, 1063-1064.
https://doi.org/10.1039/A903063H - Hiroki Oguri, Shin-ichiro Tanaka, Shojiro Hishiyama, Tohru Oishi, Masahiro Hirama,* Takeshi Tsumuraya, Yoshihisa Tomioka, Michinao Mizugaki
“Designed Hapten Aimed at Anti-ciguatoxin Monoclonal Antibody: Synthesis, Immunization and Discrimination of the C2 Configuration”
Synthesis 1999, Special Issue, 1431-1436.
https://doi.org/10.1055/s-1999-3646 - Go Hirai, Hiroki Oguri, Masahiro Hirama*
“Synthetic Study of Zoanthamine Alkaloids: The C-Ring Model Possessing Three Consecutive Quaternary Carbons”
Chem. Lett. 1999, 141-142.
https://doi.org/10.1246/cl.1999.141 - Hiroki Oguri, Shojiro Hishiyama, Oki Sato, Tohru Oishi, Masahiro Hirama,* Michio Murata, Takeshi Yasumoto, Nobuyuki Harada
“Synthetic Study of Ciguatoxin: Absolute Configuration of the C2 Hydroxyl Group”
Tetrahedron 1997, 53, 3057-3072.
https://doi.org/10.1016/S0040-4020(97)00071-9 - Masayuki Satake, Akio Morohashi, Hiroki Oguri, Tohru Oishi, Masahiro Hirama,* Nobuyuki Harada, Takeshi Yasumoto*
“Absolute Configuration of Ciguatoxin”
J. Am. Chem. Soc. 1997, 119, 11325-11326.
https://doi.org/10.1021/ja972482t - Hiroki Oguri, Shojiro Hishiyama, Tohru Oishi, Masahiro Hirama*
“Enantio-Controlled Synthesis of the AB-Ring Moiety of Ciguatoxin”
Synlett 1995, 1252-1254.
https://doi.org/10.1055/s-1995-5259 - Tohru Oishi, Hiroki Oguri, Masahiro Hirama*
“Asymmetric Baylis-Hillman Reactions Using Chiral 2,3-Disubstituted 1,4-Diazabicyclo[2.2.2]octanes Catalysts under High Pressure Conditions”
Tetrahedron: Asymmetry1995, 6, 1241-1244.
https://doi.org/10.1016/0957-4166(95)00153-G


