Research
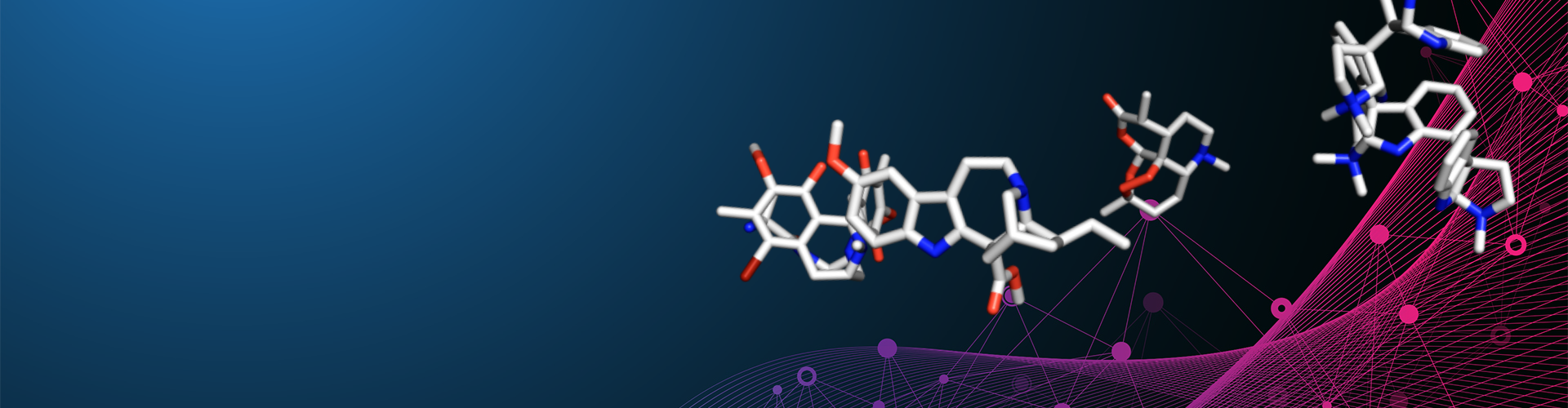
Modular assembly line synthesis of skeletally diverse molecules (ref. 1–5)
Organic synthesis that diversifies the three-dimensional structures through generation of diverse molecular scaffolds is becoming more important as an innovative technology toward the creation of functional substances based on regulation at atomic/molecular levels. We are exploring synthetic approaches that not only mimic biosynthetic processes generating a wide variety of secondary metabolites but also redesign the modular divergent assembly lines. Our synthetic campaigns aim to develop a concise and versatile synthetic process allowing systematic generation of “scaffold variations” through programmable manipulations of a common multipotent intermediate. These investigations are formulating advanced synthetic strategies to gain expeditious and cost-effective access to the natural product-relevant chemical space with diversification of skeletal, stereochemical, and functional group properties.
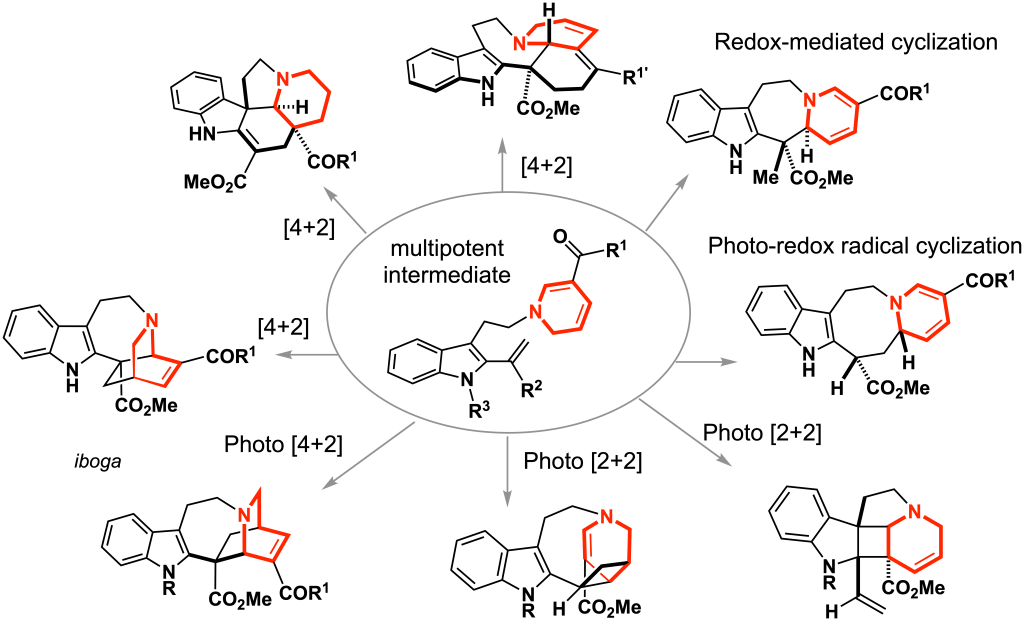
Redesigning Biosynthetic Process: Chemo-enzymatic Hybrid Synthesis (ref. 6–7)
Plants and microorganisms have evolved the enzymatic machinery to efficiently biosynthesize natural products under physiological conditions. Our approach focuses on the merger of the in vitro engineered biosynthesis and the precise organic synthesis to facilitate the generation of natural product-based complex molecules towards drug discovery and chemical genetic investigations. By streamlining enzymatic reactions and chemical manipulations, we developed a chemoenzymatic hybrid process that allowed very rapid and operationally simple access to the densely functionalized pentacyclic alkaloidal skeleton within a single day from two simple synthetic substrates. The judicious choice of the designer substrates for the enzyme SfmC allowed divergent total syntheses of saframycins and jorunnamycins in just 4–5 pot, which could be a versatile platform for the collective synthesis of natural products and their variants.
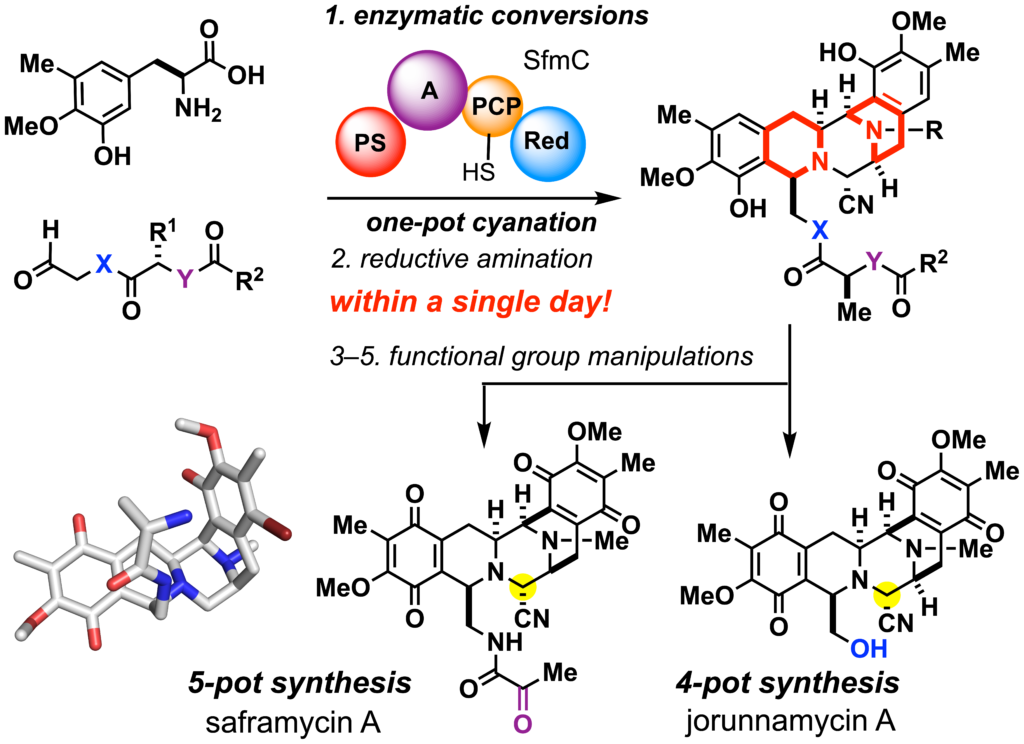
Chemical biology utilizing covalent ligands (ref. 8–9)
Artemisinin and its derivatives, the active ingredients of traditional Chinese medicine, have revolutionized the malaria chemotherapy. Reductive cleavage of the peroxide bridge by intracellular heme iron generates carbon radical species responsible for the formation of a covalent linkage with biomolecules and the enhancement of oxidative stress. The 6-aza-artemisinins were designed by replacing a stereogenic carbon center at C6 with a nitrogen, which allowed both structural modification of the hitherto unexplored C-ring and concise de novo synthesis. By exploiting the natural products variants, we will streamline concise synthesis, screening, and identification of the covalent complexes composed of the synthetic ligands and biomacromolecules. These efforts are expected to facilitate the development of lead candidates for the treatment of infectious diseases and cancers.
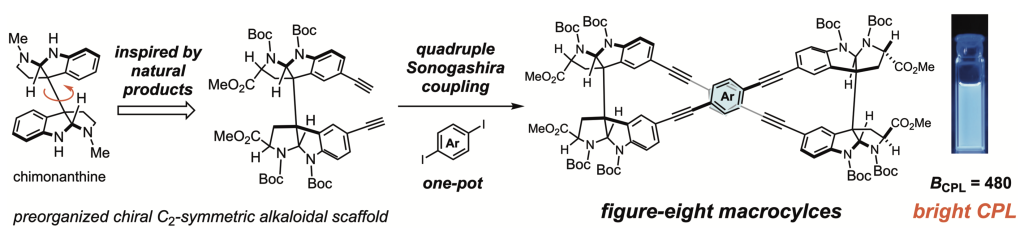
Supramolecular assemblies of natural product-based scaffolds (ref. 10)
In efforts to generate self-assembling nanostructures, we paid attention to the precise molecular recognition capabilities of natural products with dense arrays of sp3 stereogenic centers and various functional groups. The C2-symmetric alkaloidal skeleton has been demonstrated to be a versatile chiral scaffold for supramolecular chemistry, capable of generating configurational variations of the sp3 stereogenic centers and thereby customizing the conformational flexibility, chiroptical property and self-assembling behavior. We are developing the natural product-based versatile scaffolds that project multiple functional units with systematic diversification of the spatial arrangements. Supramolecular assemblies of the synthetic mid-sized molecules will be investigated to improve their sensing functions.
References
- Biogenetically inspired synthesis and skeletal diversification of indole alkaloids. H. Mizoguchi, H. Oikawa, H. Oguri. Nat. Chem. 2014, 6, 57–64.
- Zn(OTf)2-mediated annulations of N-propargylated tetrahydrocarbolines: divergent synthesis of four distinct alkaloidal scaffolds. S. Yorimoto, A. Tsubouchi, H. Mizoguchi, H. Oikawa, Y. Tsunekawa, T. Ichino, S. Maeda, H. Oguri. Chem. Sci. 2019, 10, 5686–5698.
- Synthesis of a Halicyclamine-type Macrocyclic Scaffold via Biomimetic Transannular Cyclization. T. Wayama, H. Oguri. Org. Lett. 2023, 25, 3596–3601.
- Synthetic Modulation of an Unstable Dehydrosecodine-type Intermediate and Its Encapsulation into a Confined Cavity Enable Its X-ray Crystallographic Observation. G. Tay, T. Wayama, H. Takezawa, S. Yoshida, S. Sato, M. Fujita, H. Oguri. Angew. Chem. Int. Ed. 2023, 62, e202305122.
- Direct photochemical intramolecular [4 + 2] cycloadditions of dehydrosecodine-type substrates for the synthesis of the iboga-type scaffold and divergent [2 + 2] cycloadditions employing micro-flow system. G. Tay, S. Nishimura, H. Oguri Chem. Sci. 2024, 15, 15599–15609 (Edge Article).
- Chemo-enzymatic Total Syntheses of Jorunnamycin A, Saframycin A, and N-Fmoc Saframycin Y3. R. Tanifuji, K. Koketsu, M. Takakura, R. Asano, A. Minami, H. Oikawa, H. Oguri. J. Am. Chem. Soc. 2018, 140, 10705–10709.
- Chemo-enzymatic total syntheses of bis-tetrahydroisoquinoline alkaloids and systematic exploration of the substrate scope of SfmC. R. Tanifuji, N. Haraguchi, H. Oguri, Tetrahedron Chem. 2022, 1, 100010.
- Rapid and Systematic Exploration of Chemical Space Relevant to Artemisinins: Anti-malarial Activities of Skeletally Diversified Tetracyclic Peroxides and 6-Aza-artemisinins. J. Org. Chem. 2020, 85, 9694–9712.
- Synthesis and Structural Diversification of Artemisinins Towards the Generation of Potent Anti-malarial Agents H. Oguri. Chem. Lett. 2021, 50, 924–937 (highlighted review).
- Rapid Synthesis of Chiral Figure-Eight Macrocycles Using a Preorganized Natural Product-Based Scaffold. T. Honda, D. Ogata, M. Tsurui, S. Yoshida, S. Sato, T. Muraoka,. Y. Kitagawa, Y. Hasegawa, J. Yuasa, H. Oguri Angew. Chem. Int. Ed. 2024, e202318548.


